Rare-earth half-sandwiches prove rewarding
The chemical frameworks of ’natural products’—molecules generated by biological organisms—have inspired many of today’s most potent pharmaceuticals. But the complexity of these compounds makes time-consuming tricks necessary to produce them at large scales. Bing-Tao Guan and Zhaomin Hou from the RIKEN Advanced Science Institute in Wako, however, have developed a rare-earth catalyst system that promises to make natural product synthesis significantly easier by enabling direct modification of aromatic pyridine compounds.
Pyridine, a benzene-like ring that contains nitrogen and five carbon–hydrogen (C–H) atoms, is a chemical structure found in many natural products. Ideally, chemists would insert double-bonded olefins into pyridine’s C–H groups to synthesize new medicinal compounds. But this approach is rarely viable owing to a lack of efficient and selective catalysts.
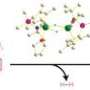
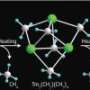
The researchers envisaged that their ‘half-sandwich’ rare-earth catalysts, which they have previously used for olefin polymerization, might offer unprecedented control over this transformation. These molecules are named after their shape, in which elements such as scandium (Sc) center above a flat pentagonal ring. They can both dehydrogenate pyridine’s C–H bonds and promote olefin insertion—two critical features in making pyridine modification a success, Hou notes.
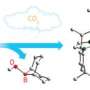
When the researchers mixed ethylene gas with a pyridine derivative and an Sc half-sandwich catalyst, they discovered that direct olefin insertion occurred at almost quantitative yields. Crucially, the researchers found that this catalysis was highly selective: the C–H bond addition occurred exclusively at a so-called ortho site adjacent to pyridine’s nitrogen atom (Fig. 1). “Selectivity is one of the most important factors for organic synthesis,” notes Guan.
Mechanistic experiments revealed that the selectivity arose from preferential binding of the rare earth to pyridine’s nitrogen atom—an action that simultaneously stabilizes the catalytic intermediate and activates the ortho-C–H bond. After the insertion of the olefin into the rare earth–pyridine bond, the reactive catalyst dehydrogenated another pyridine molecule. This action produced the newly modified pyridine derivative and regenerated the catalytic intermediate.
The researchers also found that they could tune the activity and selectivity of these catalysts by changing the central rare-earth of the half-sandwich complex. For example, switching to a rare-earth with a large ionic radius, such as yttrium (Y), enabled them to perform the first selective insertion of bulky styrene derivatives into an ortho-C–H bond of pyridine molecules. Hou and colleagues are hopeful that these versatile catalysts can yield similarly atom-efficient protocols with other synthetic reactions in the future.
More information:
Guan B.-T. & Hou, Z. Rare-earth-catalyzed C–H bond addition of pyridines to olefins. 133, 18086–18089 (2011). article
Nishiura, M. & Hou, Z. Novel polymerization catalysts and hydride clusters from rare-earth metal dialkyls. 2, 257–268 (2010).
Journal information: Journal of the American Chemical Society , Nature Chemistry
Provided by RIKEN