Cells in a tight spot
Migrating cells must overcome physical barriers such as tight pores in finely meshed tissues. A recent study by a team of LMU biophysicists provides a new theory to describe how cells manoeuvre such confining environments.
In the human body, there is a constant motion of migrating cells. Immune and cancer cells are especially motile, and are able to make their way through various barriers and thinly meshed tissue. A collaboration of experts in theoretical and experimental biophysics led by Prof. Chase Broedersz and Prof. Joachim R盲dler at LMU Munich has now proposed a new way of studying the migration of confined cells using a data-driven approach. The results are published online in Nature 糖心视频ics.
Squeezing back and forth between the two islands
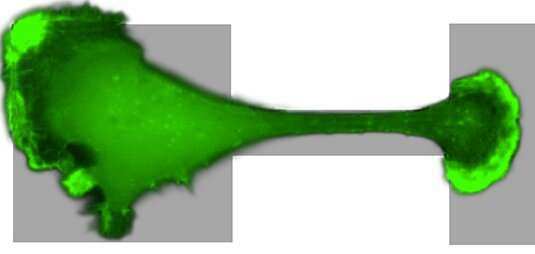
The key to their approach was to study a migrating cell in an artificial confining micro-environment. This micro-environment is made up of two islands on which a cell can comfortably sit, which are connected by a narrow bridge. They are coated with a protein to which the cell can adhere, while the surroundings are not accessible to the cell. The constriction forms a hurdle for the migrating cell, which has to squeeze its way across. Using time-lapse microscopy, the researchers monitored how the cells move around: Breast tissue cells don't just stay put, they frantically squeeze back and forth between the two islands. By watching hundreds of cells migrating on these micro-patterns, the team revealed the dynamics of how cells overcome such physical obstacles.
Crucial for the success of this study was the close collaboration between theory and experiment. "We made sure that the design of the confining environment in which the cells migrate is as simple and controllable as possible," Joachim R盲dler explains. "This allows us to use a big-data approach."
Filtering out fluctuations
The theoretical model the biophysicists propose is an equation of motion. This is similar in spirit to the equations that describe many physical systems, such as the motion of the planets around the sun. Cells however are much smaller and their motion is strongly affected by inherent fluctuations. "Using our model, we were able to disentangle the predictable, deterministic components from the random part of the motion, the fluctuations," Chase Broedersz explains. "This allows us to understand how cells can reliably perform migration tasks, despite all these random influences."
Having filtered out the fluctuations in the cell's behaviour, the scientists discovered that breast cancer cells and healthy breast cells have different motility behaviours. "Our data-driven approach combined with artificial micro-patterns allows us to reveal characteristic features of the cells," says David Br眉ckner, the first author of the study. "The inferred model therefore provides a 'motility fingerprint' that distinguishes different cell types."
Chase Broedersz concludes: "Our new approach describes confined cell migration using dynamical systems theory and shows how cells adapt to confined environments. This could also have applications for the quantitative assessment of cell behaviour in more complex biological environments."
More information: David B. Br眉ckner et al. Stochastic nonlinear dynamics of confined cell migration in two-state systems, Nature 糖心视频ics (2019).
Journal information: Nature 糖心视频ics
Provided by Ludwig Maximilian University of Munich