June 3, 2019 report
Chemists show that the catalytic range of enzymes can be enlarged

Bob Yirka
news contributor
A team of chemists at the University of Manchester has found a way to incorporate an abnormal residue into an enzyme to show how the catalytic range of enzymes can be enlarged. In their paper published in the journal Nature, the group describes expanding the range of catalytic enzymes that could be used to provide a broader variety of side chains for catalysis—by using an extended "alphabet" of amino acids. Adam Nelson, with the University of Leeds, has published a News and Views Research discussing the work by the team in the same journal issue.
As Nelson notes, there are not very many natural amino-acid residues that can be used by enzymes to catalyze reactions—he notes that there are only 20 kinds that can be used to build such enzymes. This paucity of options has led researchers to consider whether the range of amino acids that could be used by enzymes to allow for more catalytic reactions could be broadened—perhaps by using what the researchers describe as an "alphabet" of amino acids that deliver a broader variety of side chains that can be used for catalysis. In this new effort, the researchers employed such an extended alphabet of amino acids to expand the range of possibilities. They report that in so doing, they were able to construct an enzyme using members of an unnatural catalytic group and showed that doing so could lead to improvements using an approach called directed evolution.
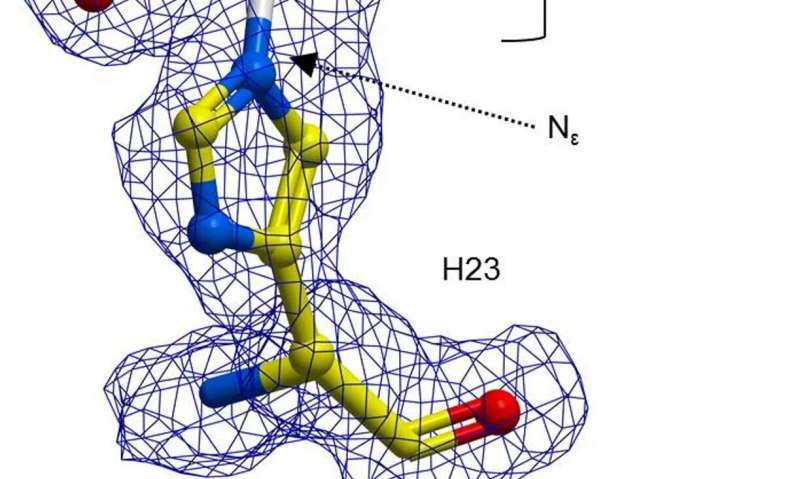
In their work, the researchers opted to remodel an enzyme to make it a more effective catalyst—they started by noting that a histidine amino-acid residue in the enzyme BH32 can form an intermediate acyl-enzyme compound. That intermediate was hydrolyzed to create a product from the reaction, but the result was not as expected. The team next used directed evolution to optimize the role of the Nδ-methylhistidine. That involved a number of approaches to force mutations. As a result, the team discovered a variant called OE1.3, which testing showed was more efficient. The team carried on with more directed evolution and eventually arrived at OE1.4—an enzyme that demonstrated improved catalytic activity.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Ashleigh J. Burke et al. Design and evolution of an enzyme with a non-canonical organocatalytic mechanism, Nature (2019).
Journal information: Nature
© 2019 Science X Network