How a bacterial pathogen that causes dysentery manipulates molecular activity to assure its survival

Virginia Tech researchers have learned how bacteria manipulate molecules to infect the host organism. Daniel Capelluto and his research team have discovered the mechanism by which the bacterial pathogen Shigella flexneri, the causative agent of dysentery, manipulates molecular activity to assure its survival against its host's natural defenses.
Their findings were recently in the journal Structure.
"This infection strategy may be employed by other bacteria, making this research a potential foundation for understanding the molecular mechanisms underlying various bacterial infections," said Capelluto, associate professor of biological sciences.
By understanding the specific manner in which a typical bacterium progresses, researchers can more precisely target preventive measures that will interrupt that process.
To survive, bacteria infect a host by replicating themselves, infecting cells, and then exiting those infected cells. A typical example of this process is seen in Shigella flexneri, a bacterium transmitted through contaminated water or food and that targets the intestinal lining.
According to Capelluto, dysentery is prevalent in low- and middle-income countries, especially among children under 5 years old, and is responsible for 160,000 deaths worldwide each year.
"Pathogens such as bacteria infect cells and they change the metabolism or the behavior of the cell they are infecting to prepare for their invasion," said Capelluto, an affiliate with the Fralin Life Sciences Institute. "The bacteria release a bunch of different proteins, and those proteins begin to mess up the host to make sure the bacteria can survive under the hostile environment."

Bacterial proteins disrupt the homeostasis, or balance, of the metabolism in the host, which causes an acidic environment and produces a large amount of lipids that is usually present in traces in the host cell.
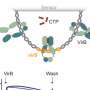
In a healthy organism, certain proteins, TOM1 and TOLLIP, serve the function of delivering no longer needed membrane proteins for degradation. However, when disrupted by a bacterial infection and under acidic conditions, TOM1 and possibly TOLLIP are intracellularly sequestered by binding to the bacterially produced lipid, promoting the survival of the infected cell so the bacterium can progress its infection cycle.
"Using high resolution biochemical and biophysical tools, we identified the lipid binding site in TOM1 and show evidence that this mechanism prevents TOM1 from its normal function," Capelluto said.
Locating the site where the critical binding occurs is fundamental to understanding this bacterial infection pathway, and it has the potential to provide insight to unravel other bacterial infection pathways.
Going forward, Capelluto aims to continue this research on another level.
"It would be nice to do some sort of studies at the cellular level, and that's what we plan to do next," Capelluto said.
More information: Wen Xiong et al, An internal linker and pH biosensing by phosphatidylinositol 5-phosphate regulate the function of the ESCRT-0 component TOM1, Structure (2024).
Journal information: Structure
Provided by Virginia Tech