Amorphization alters nanocatalyst properties: Research shows the impact of structural disorder

A research team studied how iridium and palladium nanoparticles can change the properties of catalysts with minor degradation and transition to an amorphous state. The team includes Skoltech and Khakassian State University researchers led by Skoltech Professor Alexander Kvashnin, a Doctor of Sciences in ������Ƶics and Mathematics.
The are published in the Journal of Catalysis. They provide insights into important catalysts for oxygen and hydrogen evolution reactions, as well as oxygen reduction reactions.
The transition from microparticles to nanoparticles leads to large changes in the physical and chemical properties of the material. In nanoparticles, the number of atoms on the surface and in the particle is practically the same.
Quantum effects, which are described by the laws of quantum physics, have the greatest influence on the properties of particles. Nanoparticles are used almost everywhere—in drug delivery systems, LED screens, chemical fertilizers, and so on.
"Palladium and iridium nanoalloys are important catalysts for many organic reactions. They are also used in the oxidation of carbon monoxide. Understanding what takes place at the atomic level is crucial, as it is difficult to determine the surface composition of these bimetallic nanoparticles in a real experiment, whether they form a shell on their surface, which type of shell is better in terms of energy," commented Professor Alexander Kvashnin from the Skoltech Energy Transition Center.
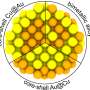
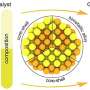
The geometric structure is essential to comprehending the catalytic properties of nanocatalysts. In the study, the authors examined core-shell iridium-palladium nanoparticles with different chemical ordering: iridium-core, palladium-shell and vice versa, as well as iridium and palladium alloys.
The research investigated the effect of the composition, type of structure (crystalline or amorphous), and local atomic environment of nanoparticles with a diameter of 2 nm on the electronic properties and charge distribution.
"The melting point of nanoparticles is significantly lower than that of bulk palladium or iridium. If the catalytic reaction takes place at high temperatures, the particles can melt—transform into an amorphous structure," added Ilya Chepkasov, the study lead author and a senior research scientist at the Skoltech Energy Transition Center.
"Most of the research focuses on the properties of crystal structures. Our idea was to see how the properties of the catalyst would change if the particle went into an amorphous state."
The authors concluded that the type of core-shell nanoparticles together with the thickness of the shell with respect to the core drastically affects the surface charge.
Nanoparticles where the iridium core is coated by an atomic-thick palladium shell show a significant excess of electrons flowing from the iridium core to the surface, forming a negative charge on it. At the same time, the type of nanoparticle structure—crystalline or amorphous—has virtually no effect on the surface charge.
More information: Ilya V. Chepkasov et al, Adsorption properties of crystalline and amorphous PdIr nanoparticles. A systematic first-principles study, Journal of Catalysis (2025).
Provided by Skolkovo Institute of Science and Technology