Electrosynthesis of urea from flue gas achieves high efficiency with no ammonia byproducts

Urea, with the formula CO(NH2)2, is a chemical compound that is widely used in a range of sectors, including manufacturing, agriculture and various industries. Conventionally, this compound is produced via a two-step process that entails the synthesis of ammonia from nitrogen (N鈧�) and its subsequent reaction with carbon dioxide (CO鈧�).
This reaction occurs at high temperatures and under high pressure, leading to the formation of a compound called ammonium carbamate. This compound is then decomposed at lower pressures, which ultimately produces urea and water.
Traditional processes for producing urea are very energy intensive, meaning that to produce desired amounts of urea they consume a lot of electrical power. Over the past few years, some engineers have thus been trying to devise more energy-efficient strategies to synthesize urea.
One possible approach could be to directly synthesize urea from CO鈧� and N鈧� using electrolyzers, devices that utilize electricity to facilitate desired chemical reactions. So far, the use of these devices to synthesize urea has proved difficult, as unsought side reactions within the devices often produce other compounds instead.
Researchers at Sun Yat-Sen University in China recently introduced a new strategy to synthesize pure urea from pre-treated flue gas, a waste gas emitted from industrial processes, in a proton-limited environment attained using an electrolyzer that integrates a solid-state electrolyte. Their paper, in Nature 糖心视频, could open new valuable opportunities for the energy-efficient production of urea on a large scale.
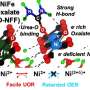
"The electrosynthesis of pure urea via the co-reduction of CO2 and N2 remains challenging," wrote Yan-Chen Liu, Jia-Run Huang and their colleagues in their paper. "We show that a proton-limited environment established in an electrolyzer equipped with a porous solid-state electrolyte, devoid of an aqueous electrolyte, can suppress the hydrogen evolution reaction and excessive hydrogenation of N2 to ammonia.
"This can instead be conducive to the C鈥揘 coupling of *CO2 with *NHNH (the intermediate from the semi-hydrogenation of N2), thereby facilitating the production of urea."
The new strategy to synthesize urea introduced by this research team primarily relies on the creation of a proton-limited environment, a condition in which hydrogen ions (i.e., protons) are scarce. This condition was successfully realized using an electrolyzer that contains a porous solid-state electrolyte.
"By using nanosheets of an ultrathin two-dimensional metal鈥揳zolate framework with cyclic heterotrimetal clusters as catalyst, the Faradaic efficiency of urea production from pretreated flue gas (which contains mainly 85% N2 and 15% CO2) is as high as 65.5%, and no ammonia and other liquid products were generated," wrote Liu, Huang and their colleagues.
"At a low cell voltage of 2.0鈥塚, the current can reach 100鈥塵A, and the urea production rate is as high as 5.07鈥塯鈥塯cat鈭�1鈥塰鈭�1 or 84.4鈥塵mol鈥塯cat鈭�1鈥塰鈭�1. Notably, it can continuously produce 6.2鈥墂t% pure urea aqueous solution for at least 30鈥塰, and about 1.24鈥塯 pure urea solid was obtained."
In the team's initial experiments, their strategy enabled the continuous production of high-purity urea with no ammonia byproducts, while consuming less energy than conventional urea synthesis approaches. In the future, it could be tested further and implemented on a large scale, potentially enabling the greener and cost-effective production of urea on a large scale.
"The use of pretreated flue gas as a direct feedstock significantly reduces input costs, and the high reaction rate and selectivity contribute to a reduction in system scale and operational costs," wrote the researchers.
More information: Yan-Chen Liu et al, Electrosynthesis of pure urea from pretreated flue gas in a proton-limited environment established in a porous solid-state electrolyte electrolyser, Nature 糖心视频(2025).
Journal information: Nature 糖心视频
漏 2025 Science X Network