Light-as-a-feather nanomaterial extracts drinking water from air

Lisa Lock
scientific editor

Robert Egan
associate editor

An international scientific collaboration has developed a novel nanomaterial to efficiently harvest clean drinking water from water vapor in the air. The nanomaterial can hold more than three times its weight in water and can achieve this far quicker than existing commercial technologies, features that enable its potential in direct applications for producing potable water from the air.
The collaboration is led by the Australian Research Council Center of Excellence for Carbon Science and Innovation (ARC COE-CSI) UNSW Associate Professor Rakesh Joshi and Nobel Laureate Professor Sir Kostya Novoselov. Prof Joshi is based at the School of Materials Science and Engineering, University of New South Wales (UNSW). Prof Novoselov is based at the National University of Singapore.
A estimates that 2.2 billion people lack safely managed drinking water.
On Earth, there are about 13 million gigaliters of water suspended in the atmosphere (Sydney harbor holds 500 gigaliters). While that is only a fraction of the total water on Earth, it still amounts to a substantial source of fresh water.
"Our technology will have application in any region where we have sufficient humidity but limited access to or availability of clean potable water," Dr. Joshi says.
Prof Novoselov says, "This is an excellent example of how interdisciplinary, global collaboration can lead to practical solutions to one of the world's most pressing problems—access to clean water."
The research is in the Proceedings of the National Academy of Sciences.
Finding magic in the bonding
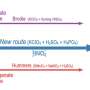
The novel nanomaterial is based on the well-studied form of the graphene oxide, which is a single-atom-thick carbon lattice functionalized with oxygen-containing groups. Graphene oxide has good water adsorption properties, which are properties that enable water to bond to the surface of a material.
Calcium also has good water adsorption properties. The research team decided to see what happened if you intercalate calcium ions (Ca2+) into the graphene oxide.
What happened was unexpected.
An important characteristic of materials that effectively adsorb water is strong hydrogen bonds between the water and the material it adsorbs onto, something that graphene oxide and calcium each have. The stronger the hydrogen bond, the more a material can adsorb water.
But some magic happens when you intercalate calcium to the oxygen in the graphene oxide.
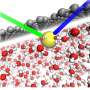
In calcium-intercalated graphene oxide, it is the synergy between calcium and oxygen that facilitates the extraordinary adsorption of water.
What the research team discovered is that the way the calcium coordinates with the oxygen in the graphene changes the strength of the hydrogen bonds between the water and the calcium to make those bonds even stronger.
"We measured the amount of water adsorbed onto graphene oxide by itself and we measured X. We measured the amount of water adsorbed onto calcium itself and we got Y. When we measured the amount of water adsorbed onto the calcium-intercalated graphene oxide we got much more than X+Y. Or it is like 1+1 equals a number larger than 2," says Xiaojun (Carlos) Ren, UNSW School of Materials Science and Engineering and first author on the paper.
"This stronger-than-expected hydrogen bonding is one of the reasons for the material's extreme ability to adsorb water," he says.
It's also light as a feather
There was one more design tweak the team did to enhance the material's water-adsorbing ability—they made the calcium-intercalated graphene oxide in the form of an aerogel, one of the lightest solid materials known.
Aerogels are riddled with micro- to nanometer-sized pores, giving them a massive surface area, which helps this aerogel form adsorb water far quicker than the standard graphene oxide.
The aerogel also gives the material sponge-like properties that make the desorption process, or release of the water from the membrane, easier.
"The only energy this system requires is the small amount needed to heat the system to about 50 degrees to release the water from the aerogel," says Professor Daria Andreeva, the co-author of the paper.
The power of the supercomputer
The research is based on experimental and theoretical work that relied on the Australian National Computational Infrastructure (NCI) supercomputer in Canberra.
Professor Amir Karton from the University of New England led the computational work to provide the crucial understanding of the underlying mechanism.
"The modeled simulations done on the supercomputer explained the complex synergistic interactions at the molecular level, and these insights now help to design even better systems for atmospheric water generation, offering a sustainable solution to the growing challenge of fresh water availability in regional Australia and in water-stressed regions across the globe," says Professor Karton.
The power of science without borders
This is still a fundamental research discovery that needs further development. Industry have collaborated on this project to help scale up this technology and develop a prototype for testing.
"What we have done is uncover the fundamental science behind the moisture adsorption process and the role of hydrogen bonding. This knowledge will help provide clean drinking water to a large proportion of those 2.2 billion people that lack access to it, demonstrating the societal impact by collaborative research from our Center," says COE-CSI Director and one of the co-authors on the paper, Professor Liming Dai.
The research is a global collaboration between research groups from Australia, China, Japan, Singapore and India.
More information: Xiaojun Ren et al, Synergetic hydrogen-bond network of functionalized graphene and cations for enhanced atmospheric water capture, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences