Nanobody-based 3D immunohistochemistry allows rapid visualization in thick tissue samples

Lisa Lock
scientific editor

Robert Egan
associate editor

Three-dimensional immunohistochemistry (3D-IHC) has transformed our ability to visualize the spatial arrangement of cells and molecules in intact tissues. However, traditional methods are often time-consuming and suffer from poor antibody penetration, which limits their effectiveness in deep tissues. This bottleneck has posed significant challenges in neuroscience, pathology, and biomedical imaging, where rapid and detailed mapping of large tissue volumes is essential.
To address these issues, researchers at Juntendo University in Tokyo, Japan, Assistant Professor Kenta Yamauchi and Professor Hiroyuki Hioki, have developed a new 3D-IHC technique that dramatically improves the speed, depth, and sensitivity of immunolabeling in thick tissue samples.
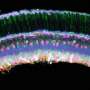
The study—titled "A three dimensional immunolabeling method with peroxidase-fused nanobodies and fluorochromized tyramide-glucose oxidase signal amplification," on June 18, 2025, in Communications Biology—employs a method called POD-nAb/FT-GO 3D-IHC, which combines nanobodies fused with peroxidase (POD-nAbs) and a fluorescent signal amplification system known as Fluorochromized Tyramide-Glucose Oxidase (FT-GO).
This approach enables the detection of proteins in 1-mm-thick brain tissue within three days, a fraction of the time required by conventional techniques.
"POD-nAbs, camelid nAbs fused with POD, enhanced immunolabeling depth and enabled sensitive detections by combining with our original fluorescent tyramide signal amplification system, FT-GO," said Dr. Yamauchi.
The researchers began by addressing a long-standing limitation in 3D tissue immunohistochemistry: the poor penetration of conventional immunoglobulin antibodies, which are large in size (~150 kDa). Nanobodies, derived from camelid antibodies, are only ~15 kDa and therefore diffuse much more efficiently into thick tissues.
The team engineered nanobodies fused with horseradish peroxidase, which catalyzes the deposition of fluorescent tyramides via the FT-GO system, enabling high-density labeling of target molecules.
After optimizing tissue permeabilization with a urea-based solution called ScaleA2, the researchers demonstrated that POD-nAbs could achieve nearly uniform labeling across the full depth of 1-mm mouse brain slices. In contrast, conventional antibodies only labeled the tissue periphery. This depth advantage was evident in labeling both exogenously expressed proteins like EGFP and tdTomato, as well as endogenous targets like integrin alpha M/cluster of differentiation molecule 11b, a marker of microglia.
"Quenching of POD activity with NaN3 allowed for multiplex immunolabeling of 3D tissues by POD-nAb/FT-GO reaction, greatly improving versatility," said senior author Dr. Hioki.
The technique also proved effective in a disease model. In brain slices from Alzheimer's disease model mice, the method revealed activated microglia clustering around beta-amyloid plaques—an important hallmark of neuroinflammation and disease progression. This shows promise for applying the method in studying neurodegenerative diseases, tumor microenvironments, and immune cell dynamics in tissues.
Compared to standard fluorescent protein imaging and direct labeling with synthetic fluorophore-conjugated nanobodies, the POD-nAb/FT-GO protocol provided up to nine-fold greater signal intensity at tissue depths of 500 microns. This substantial signal amplification reduces the need for prolonged imaging sessions, making the method particularly suited for high-throughput analysis.
Despite its advantages, the method has some limitations. The authors note that signal homogeneity decreases in tissues thicker than 1 mm. Additionally, quantitative comparisons of antigen expression are challenging due to the non-linear nature of enzymatic signal amplification. Another hurdle is the limited availability of nanobodies suitable for histochemical labeling, though this is expected to improve as more nanobody sequences become publicly available.
Still, the researchers are optimistic about the broader impact of their technique. By overcoming major barriers in antibody penetration and signal detection, POD-nAb/FT-GO 3D-IHC could set a new standard in volumetric imaging of biological tissues. This innovative protocol not only opens new doors for basic neuroscience but also holds potential for advancing clinical diagnostics and drug discovery. With growing demand for rapid, high-resolution tissue imaging, particularly in brain research and cancer biology, the method provides a timely and scalable solution.
More information: Kenta Yamauchi et al, A three dimensional immunolabeling method with peroxidase-fused nanobodies and fluorochromized tyramide-glucose oxidase signal amplification, Communications Biology (2025).
Journal information: Communications Biology
Provided by Juntendo University Research Promotion Center