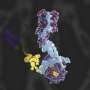
Model for DBHS mediated telomerase recruitment to telomeres. Credit: Nature Communications (2025). DOI: 10.1038/s41467-025-60924-w
A study by Children's Medical Research Institute (CMRI) researchers reveals a new group of proteins that guide the powerful enzyme telomerase, opening potential doors to novel treatments for cancer, aging, and genetic diseases.
Telomerase, a crucial enzyme that keeps our chromosomes intact during cell division, has long fascinated scientists for its dual role in both promoting healthy aging and enabling the uncontrolled growth of cancer cells.
Telomerase adds DNA to the ends of chromosomes (telomeres) to protect them from damage. It's essential in stem cells and some immune cells but is often hijacked by cancer cells to avoid aging and death. Researchers at CMRI have now identified a new set of proteins that play a vital role in controlling this enzyme.
In a recent study in Nature Communications, researchers have discovered that a group of proteins known as the DBHS protein family (including NONO, SFPQ, and PSPC1) are essential for transporting telomerase to where it's needed most—the protective ends of chromosomes called telomeres.
"Our findings show that these proteins act like molecular traffic controllers, making sure telomerase reaches the right destination inside the cell," said Dr. Alexander Sobinoff, the study's lead author. "Without these proteins, telomerase can't properly maintain telomeres, a finding which has significant implications for healthy aging and cancer progression."
Key discoveries
- The DBHS proteins interact directly with telomerase, helping it leave specific compartments in the nucleus and travel to the chromosome ends.
- Each of the three proteins plays a unique, non-redundant role in telomere maintenance.
- Prolonged disruption of these proteins in cancer cells prevents telomerase from maintaining telomeres, leading to dramatic shortening of chromosome ends, providing a strategy for preventing the relentless growth of cancers.
Telomerase is often hyperactive in cancer cells, allowing them to multiply endlessly. On the flip side, insufficient telomerase activity is linked to rare genetic disorders, premature aging, and organ failure. Understanding how telomerase is controlled could have profound implications for treating a wide range of diseases.
Head of Children's Medical Research Institute's Telomere Length Regulation Unit, Professor Hilda Pickett, senior author of the study, said, "This discovery opens up exciting new possibilities for therapies targeting aging, cancer, and beyond."
More information: Alexander P. Sobinoff et al, NONO, SFPQ, and PSPC1 promote telomerase recruitment to the telomere, Nature Communications (2025).
Journal information: Nature Communications
Provided by Children's Medical Research Institute (CMRI)