CRISPR's efficiency triples in lab tests with DNA-wrapped nanoparticles

Sadie Harley
scientific editor

Robert Egan
associate editor
With the power to rewrite the genetic code underlying countless diseases, CRISPR holds immense promise to revolutionize medicine. But until scientists can deliver its gene-editing machinery safely and efficiently into relevant cells and tissues, that promise will remain out of reach.
Now, Northwestern University chemists have unveiled a new type of nanostructure that dramatically improves CRISPR delivery and potentially extends its scope of utility.
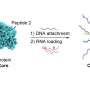
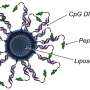
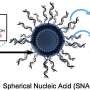
Called lipid nanoparticle spherical nucleic acids (LNP-SNAs), these tiny structures carry the full set of CRISPR editing tools—Cas9 enzymes, guide RNA and a DNA repair template—wrapped in a dense, protective shell of DNA. Not only does this DNA coating shield its cargo, but it also dictates which organs and tissues the LNP-SNAs travel to and makes it easier for them to enter cells.
In lab tests across various human and animal cell types, the LNP-SNAs entered cells up to three times more effectively than the standard lipid particle delivery systems used for COVID-19 vaccines, caused far less toxicity and boosted gene-editing efficiency threefold. The new nanostructures also improved the success rate of precise DNA repairs by more than 60% compared to current methods.
The study, "A general genome editing strategy using CRISPR lipid nanoparticle spherical nucleic acids," is published in the Proceedings of the National Academy of Sciences.
The study paves the way for safer, more reliable genetic medicines and underscores the importance of how a nanomaterial's structure—rather than its ingredients alone—can determine its potency. This principle underlies structural nanomedicine, an emerging field pioneered by Northwestern's Chad A. Mirkin and his colleagues and pursued by hundreds of researchers around the world.
"CRISPR is an incredibly powerful tool that could correct defects in genes to decrease susceptibility to disease and even eliminate disease itself," said Mirkin, who led the new study.
"But it's difficult to get CRISPR into the cells and tissues that matter. Reaching and entering the right cells—and the right places within those cells—requires a minor miracle. By using SNAs to deliver the machinery required for gene editing, we aimed to maximize CRISPR's efficiency and expand the number of cell and tissue types that we can deliver it to."
A nanotechnology and nanomedicine pioneer, Mirkin is the George B. Rathmann Professor of Chemistry at Northwestern's Weinberg College of Arts and Sciences; professor of chemical and biological engineering, biomedical engineering and materials science and engineering at the McCormick School of Engineering; professor of medicine at the Feinberg School of Medicine; executive director of the International Institute for ������Ƶ; and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
CRISPR needs a ride
When CRISPR machinery reaches its target inside a cell, it can disable genes, fix mutations, add new functions and more. But CRISPR machinery cannot enter cells by itself. It always needs a delivery vehicle.
Currently, scientists typically use viral vectors and lipid nanoparticles (LNPs) to perform this function. Naturally good at sneaking into cells, viruses are efficient, but they can cause the human body to mount an immune response, leading to painful or even dangerous side effects.
LNPs, on the other hand, are safer but inefficient. They tend to get stuck in endosomes, or compartments within the cell, where they cannot release their cargo.
"Only a fraction of the CRISPR machinery actually makes it into the cell and even a smaller fraction makes it all the way into the nucleus," Mirkin said.
"Another strategy is to remove cells from the body, inject the CRISPR components and then put the cells back in. As you can imagine, that's extremely inefficient and impractical."
A DNA-wrapped taxi
To overcome this barrier, Mirkin's team turned to SNAs, which are globular—rather than linear—forms of DNA and RNA previously invented in Mirkin's lab at Northwestern. The spherical genetic material surrounds a nanoparticle core, which can be packed with cargo.
Roughly 50 nanometers in diameter, the tiny structures possess a proven ability to enter cells for targeted delivery. Seven SNA-based therapies are already in human clinical trials, including a Phase II clinical trial for Merkel cell carcinoma being developed by Flashpoint Therapeutics, a clinical-stage biotechnology startup.
In the new study, Mirkin's team started with an LNP core carrying the CRISPR machinery inside. Then, they decorated the particle's surface with a dense layer of short strands of DNA. Because the DNA can interact with a cell's surface receptors, cells easily absorb SNAs. The DNA can also be engineered with sequences that target specific cell types, making delivery more selective.
"Simple changes to the particle's structure can dramatically change how well a cell takes it up," Mirkin said. "The SNA architecture is recognized by almost all cell types, so cells actively take up the SNAs and rapidly internalize them."
Boosted performance across the board
After successfully synthesizing LNP-SNAs with CRISPR cargo, Mirkin and his team added them to cellular cultures, which included skin cells, white blood cells, human bone marrow stem cells and human kidney cells.
Then, the team observed and measured several key factors: how efficiently the cells internalized the particles, whether the particles were toxic to cells and if the particles successfully delivered a gene.
They also analyzed the cells' DNA to determine if CRISPR had made the desired gene edits. In every category, the system demonstrated its ability to successfully deliver CRISPR machinery and enable complex genetic modifications.
Next, Mirkin plans to further validate the system in multiple in vivo disease models. Because the platform is modular, researchers can adapt it for a wide range of systems and therapeutic applications.
Northwestern biotechnology spin-out Flashpoint Therapeutics is commercializing the technology with the goal of rapidly moving it toward clinical trials.
"CRISPR could change the whole field of medicine," Mirkin said. "But how we design the delivery vehicle is just as important as the genetic tools themselves. By marrying two powerful biotechnologies—CRISPR and SNAs—we have created a strategy that could unlock CRISPR's full therapeutic potential."
More information: A general genome editing strategy using CRISPR lipid nanoparticle spherical nucleic acids, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by Northwestern University