Powering down to survive viral attack: The steps cells take to thwart replication

Sadie Harley
scientific editor

Robert Egan
associate editor

Cells have evolved ingenious ways to defend themselves from viral attack. Research from The Wertheim UF Scripps Institute sheds light on one of their sneakier strategies.
If there's a prowler trying to break into one's house, a logical reaction is to hide and call 911. Cells can do much the same. They temporarily shut down their metabolism, explains James Burke, Ph.D., an associate professor of molecular medicine at The Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology. But how?
In a study published in the journal , Burke and his team detailed the mechanics of how and when cells go dark after infection with West Nile virus.
Their discoveries challenge widely-held ideas about cells' antiviral defenses and illuminate new details about the inner workings of humans' ancient, innate immune system.
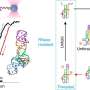
Viruses infect cells with a goal of taking control of their protein-building machinery to churn out new viral copies. Cells have learned to fight back by activating an enzyme called RNase L. These scissors-like molecules degrade the materials needed to make proteins, Burke said. Cells can remain in this "dark" mode up to 24 hours, he said.
"One of the questions we wanted to address was, 'How do cells make the decision to degrade all of their own RNA?'" Burke said.
Burke and his team found a process that was much more complex than previously known.
"Understanding this complexity is key to developing next-generation antiviral therapeutics that can combat viral infections," Burke said.
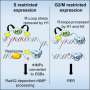
The team's experiments showed that a group of specialized proteins known as OAS 1, 2, 3 and L, short for "oligoadenylate synthetase," played a variety of roles. They found that OAS 3 proteins crowded around the foreign, double-stranded viral RNA, binding tightly in three locations. Once this binding is tight enough, the normally quiet RNase L enzyme springs into action, they found.
Curiously, they observed RNase L degrade the cell's own messenger RNA, but not the foreign viral RNA lurking within the cell. Burke's hypothesis is that some viruses have developed defensive strategies of their own, such as protecting their genetic material in sacs called vesicles. It's a subject of ongoing research, he said.
While cells are working to protect themselves, so is West Nile virus, which can lay low—too low to activate RNase L—in the first 24 hours of infection, he said.
"It's just like chess," Burke said. "The virus makes a move, the cell responds, the virus tries a new move."
Burke's team found many surprises as they exposed lung cells to West Nile virus and then, using a powerful microscope, watched the changes within, with the help of fluorescent tags.
Previously, scientists thought that the main player on the RNase L-defensive team was OAS 1, which is present in the mice used in many research studies. But Burke's team found it was the aggregation of OAS 3, an exclusively human player, that activated RNase L.
Scientists have also suspected that RNase L worked by cleaving ribosomes, organelles that are cells' protein-building factories. Burke's team showed the enzyme degraded the templates that ribosomes use to assemble proteins, the cell's messenger RNA, but not the ribosomes.
"Within 30 minutes, RNase L will degrade almost all messenger RNA in a cell," Burke said.
Also surprising, they found that sometimes, white blood cells' release of interferon was enough to defend against West Nile virus, so that RNase L need not shut down cellular protein production. That created an "aha" moment, Burke said. RNase L only turns on when the infection is beyond interferon's control.
"This completely changes how we think about this pathway's relation to interferon in human cells," he said.
Many questions remain.
The researchers want to know how the West Nile virus hides from RNaseL and whether that process can be disrupted. They want to know if RNase L dampens inflammation. And they want to know how other viruses, like SARS-CoV-2, adapt to RNaseL.
They used West Nile virus in the study to understand this fundamental biological process, but expect to see similar results with other viruses, with nuances.
"This study completely changed my understanding of the RNase L pathway," Burke said. "Like a puzzle, every time you find a new piece that fits, the picture becomes clearer."
More information: Skyler Briggs et al, Condensation of human OAS proteins initiates diverse antiviral activities in response to West Nile virus, Genes & Development (2025).
Journal information: Genes & Development
Provided by University of Florida