Creating safe medicinal molecules with sustainable electrochemistry

Gaby Clark
scientific editor

Robert Egan
associate editor

Cornell chemists have developed a way to use electrochemistry, a sustainable technique, to make chiral molecules, which occur in mirrored pairs, like human hands. Common in pharmaceuticals, chiral molecules are important to get right to be effective and safe.
"Many drug molecules are chiral—like our hands, they look similar, but one could be very effective in treating a disease while the other could be inactive or even a poison," said Song Lin, the Howard Milstein Faculty Fellow/Tisch University Professor of chemistry and chemical biology in the College of Arts and Sciences (A&S). "A lot of times, making only one of the two mirror image molecules is important in medicinal chemistry."
In some medicines, such as the pain reliever ibuprofen, one side of a chiral molecule is active and effective while the other (called its enantiomer) is present but benign, Lin said. In many other drugs, the active side is mirrored by a toxic enantiomer that must be eliminated, making chiral molecule synthesis an important objective in organic chemistry. Work on asymmetric catalysis—using chiral catalysts in a solution to induce handedness—earned Nobel Prizes in 2001 and 2021.
In "Dynamic Kinetic Resolution of Phosphines with Chiral Supporting Electrolytes," in Nature, Lin, the corresponding author, and collaborators describe how they use electrochemistry to selectively synthesize chiral compounds using the reaction's electrolytes to introduce a chiral environment, a completely new strategy for inducing chirality in electrochemistry.
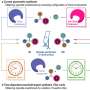
Although it has clear environmental benefits, electrochemistry, an expertise of the Lin Lab, is rarely used to create chiral molecules, Lin said, because of challenges where the solid electrode, made of metal or carbon, meets the liquid solution. The Cornell researchers overcame this problem by introducing a chiral shape to the reaction through electrolytes, usually a background element.
"Supporting electrolytes are salts added to experiments just to make sure the solution is conducting," Lin said. "They do not usually play explicit roles in the reaction. But in this work, we use electrolytes to interact with electrochemically generated molecules through simple electrostatic interactions, so that only one of the two mirror image products, the desired enantiomer, is formed."
Because the electrolyte is always there at the interface, Lin said, this technique can theoretically be generally applied to many different types of electrochemical reactions, including making drug molecules.
Chiral molecules are common in medicines because chirality is common in nature, from spiraling vines and shells to amino acids and various proteins, to the human body's protein receptors, the site of drug interaction.
"Humans are made of chiral molecules," Lin said. "Because your protein receptors are chiral, they're going to interact with chiral molecules differently."
The researchers collaborated with a lab at Brown University to observe the reaction on the molecular level using molecular dynamics simulation, an advanced computational technique.
"It is well known that the ions in the supporting electrolyte (e.g. salt), which you dissolve in the solution, become even more concentrated near the electrode surface with the opposite charge," said Yue Qi, the Joan Wernig Sorensen Professor of Engineering at Brown University. "So now you have an even higher concentration of the chiral inducer near the electrode surface, which is going to make the electrochemical reaction more effective."
This collaboration was facilitated through the National Science Foundation Center for Synthetic Organic Electrochemistry. The collaborators plan to examine the mechanism in more detail to understand exactly how it works. They also want to explore what other types of molecules they can make using chiral supporting electrolyte.
"I think this technology could, in the future, be used in industry, but going from academic discovery to industry application always takes a long time," Lin said. "It's important that NSF supports fundamental research. It allows us to discover something that might not be immediately useful but could play a big role in the long-term."
More information: Kaining Mao et al, Dynamic kinetic resolution of phosphines with chiral supporting electrolytes, Nature (2025).
Journal information: Nature
Provided by Cornell University