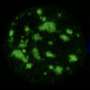
Microscopy image showing nuclear speckles (green), a mix-charged protein that is induced in a subset of cells (yellow), a specific mRNA that doesn't respond to interstasis (control—left slide) or mRNA that contains repetitive sequences (multivalent mRNA- right slide). Only the multivalent mRNA is captured into nuclear speckles in the cells that express the mix-charged protein (merged speckle—right slide). Credit: Nature (2025). DOI: 10.1038/s41586-025-09568-w
The amount of any given protein in a cell has to be controlled to keep its levels within a range required for healthy functions. This is especially important for proteins that are known to group together in liquid droplets called "condensates." These proteins generally contain flexible parts that don't have fixed 3D structures, so they can form many interactions at the same time. When these proteins accumulate, they are prone to forming large clumps called aggregates.
Proteins that have similar flexible parts tend to condense together, so their equilibrium is a collective problem for the cell. "I compare accumulation of these proteins to global warming, where we're all contributing a tiny bit too much to greenhouse gas emissions and that adds up to cause a huge problem," explains Jernej Ule, who leads a lab at the Crick. "Similarly, small increases in many individual proteins could collectively cause a big effect on their joint condensate."
Ule also leads the UK Dementia Research Institute at King's College London. He's interested in these flexible proteins because their accumulation or aggregation can be toxic to the cell, causing conditions like neurodegenerative diseases.
Aiming to discover how the cell regulates the amounts of these types of proteins, Rupert Faraway and Neve Costello Heaven in Jernej's team investigated so-called "nuclear speckles"—condensates in the nucleus that contain diverse proteins and RNAs.
Their findings, in Nature, enabled them to discover a new way for cells to maintain the equilibrium of many proteins that condense together.
Interstasis: Solving a collective problem
Faraway first used a computer program to find that many messenger molecules, called mRNAs, contain "red flags" of repeated sequences. These sequences tend to encode the flexible parts of proteins.
"Interestingly, evolution has introduced a bias toward certain genetic codons (three-letter codes) that promote repetitive sequences in mRNAs. And we've found that these sequences encode flexible parts of proteins with related functions," says Faraway. "It's this similarity in mRNA sequences that helps the cell regain equilibrium if too many of these proteins are gathered together."
For example, one of these sequences preferred by evolution encodes proteins containing regions with a mix of negative and positive charges, called "mix-charged proteins," which are found in nuclear speckles.
Costello Heaven used fluorescent tags to see what happened if large amounts of mix-charged proteins gathered in the speckles. She explains, "When the amounts of mix-charged proteins in speckles were increased, we saw a build-up of their own mRNAs also within the speckles. And this decreased further production of these proteins, promoting their collective equilibrium."
The team termed this process "interstasis": how the accumulation of various proteins in a condensate can decrease further production of the same proteins by capturing their own mRNAs into the same condensate.
Costello Heaven continues, "Interstasis is a way for the cell to regulate genes that are particularly 'dosage-dependent'—their levels have to be just right. When their mRNAs are taken into the speckles, cells can't produce more of the same protein."
Does interstasis fail in neurodegeneration?
The team's discovery has opened doors to study what happens if interstasis fails to control a build-up of these types of proteins.
"Many diseases of aging involve the build-up of proteins that group together in condensates," says Ule. "We're now exploring the role of interstasis in controlling proteins that play a role in neurodegeneration, and whether a failure of interstasis could impact aggregation of these proteins."
He concludes, "Lots of areas of biology focus on feedback loops that control a specific gene, protein or pathway. We've taken a step back and looked at the cell's capacity for maintaining equilibrium of groups of proteins that participate in many different pathways. I'm hopeful that this will inspire other researchers to explore how this type of feedback mechanism might promote equilibrium in various areas of biology."
More information: Rupert Faraway et al, Collective homeostasis of condensation-prone proteins via their mRNAs, Nature (2025).
Journal information: Nature
Provided by The Francis Crick Institute