Flexible fitting method translates high-speed atomic force microscopy images into precise protein motion models

Lisa Lock
scientific editor

Robert Egan
associate editor

High-speed atomic force microscopy (HS-AFM) is the only experimental technique to directly watch proteins in dynamic action. However, as a surface scanning technique with limited spatial resolution, HS-AFM will inevitably provide insufficient information for detailed atomistic understanding of biomolecular function. Despite previous efforts in computational modeling attempting to overcome such limitations, successful applications to retrieve atomistic-level information from measurements are practically absent.
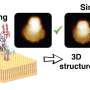
A research team led by Holger Flechsig (WPI-NanoLSI, Kanazawa University) and Florence Tama (WPI-ITbM, Graduate School of Science at Nagoya University, and R-CCS) presents a computational framework and its software implementation allowing to infer 3D atomistic models of dynamic protein conformations from AFM topography imaging.
The scientists use a new computationally efficient flexible fitting method developed by Tama's group, which models conformational dynamics of known static protein structure to identify atomistic models that best fit experimental AFM images.
They first implemented this method into the well-established BioAFMviewer software platform maintained by Flechsig's group to provide a direct workflow for applications to measure AFM imaging data. The research is in the journal ACS Nano.
The analysis of HS-AFM data for different proteins obtained by experimental collaborators evidence that flexible fitting can infer atomistic models including large-amplitude motions to significantly improve understanding of functional conformational dynamics from resolution-limited measurements.
Computational efficiency of flexible fitting within the BioAFMviewer even allows applications to large protein assemblies, as the authors show for the example of a 4 megadalton actin filament consisting of about 280,000 atoms. A remarkable achievement is the demonstration of an atomistic molecular movie of protein dynamics, involving functional conformational transitions, reconstructed from HS-AFM topographic movie data.
The unique software implementation of computationally efficient flexible fitting, integrating available structural data and molecular modeling with experiments, opens the opportunity for a broad range of applications to fully exploit the explanatory power of HS-AFM by large-scale analysis of single molecule imaging data toward better understanding biological processes at the nanoscale.
Flexible fitting is a computational method which models conformational motions of a static protein structure to dynamically steer it into conformations that best represent experimental data. The normal mode flexible fitting AFM (NMFF-AFM) method, by Tama's group, employs computational efficient iterative normal mode analysis to model large-amplitude conformational changes, which allows the identification of dynamic atomistic models that best represent measured AFM topographic images.
BioAFMviewer software
The BioAFMviewer project was initiated by Holger Flechsig in 2020, with Romain Amyot as the programming scientist, to provide a unique software platform integrating the enormous amount of available high-resolution biomolecular structure and modeling data for the analysis of resolution-limited AFM measurements.
An integrated molecular viewer for biomolecular visualization, corresponding simulation AFM, and several analysis toolboxes provide a user-friendly interactive software interface for the convenient analysis of experimental AFM data. The performance of the integrated NMFF-AFM flexible fitting method is significantly enhanced by parallelized computations executed on graphic cards.
The BioAFMviewer software is available for free download from the project website .
More information: Romain Amyot et al, Flexible Fitting to Infer Atomistic-Precision Models of Large-Amplitude Conformational Dynamics in Biomolecules from High-Speed Atomic Force Microscopy Imaging, ACS Nano (2025).
Journal information: ACS Nano
Provided by Kanazawa University