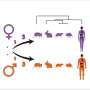
In many species, males and females differ in size. Female fruit flies are larger than males — but is that because they have more cells or because their cells are larger? Credit: Soumitra Pal
Sexual size differences are widespread in biology, yet the "how" behind them often remains vague. We asked a concrete question in a classic model organism: when female flies are larger than males, do individual organs achieve this by adding more cells, enlarging the cells they already have, or mixing both strategies—and is this consistent across the body?
Using whole-animal single-nucleus data and targeted experiments, we show the answer is nuanced: Sex-specific body proportions emerge from organ-by-organ allometry, not from a one-size-fits-all rule.
How we approached it
As part of an international team including the National Institutes of Health, U.S., along with collaborators from institutions such as Sanford Burnham Prebys, the European Molecular Biology Laboratory and the University of British Columbia, we investigated the cellular underpinnings of sexual dimorphism. The findings are on the pre-print server bioRxiv.
We mined the Fly Cell Atlas—a comprehensive, cutting-edge, single-nucleus atlas published in Science that spans ~580,000 nuclei from 15 sexed adult tissues, plus whole head and headless body—allowing us to compare male and female cell types across the entire animal using the same lens. We then validated predictions experimentally to confirm whether differences reflected changes in cell number or cell size.
What we found
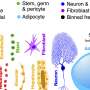
At the heart of our discovery is allometry, which means that parts of an organism scale at different rates relative to overall size (think: a violin's strings don't scale the same way as its body). Isometry would mean uniform scaling. We found that size disparities arise from tailored cellular strategies: Some organs expand by adding more cells, others by enlarging existing ones—and these strategies differ between males and females.
Take the flight muscles, essential for aerial acrobatics. In females, these muscles are larger because they begin with more precursor cells, called myoblasts, during development. Males, by contrast, make do with fewer, resulting in smaller muscles overall. This cell-number strategy highlights an efficient way to build stronger, larger structures without reinventing the wheel.
Then there's the heart, which pumps hemolymph (the fly equivalent of blood) throughout the body. Surprisingly, both sexes have the same number of cardiomyocytes—the muscle cells of the heart. Yet females end up with a larger organ because cell size, or other factors such as extracellular matrix, drive the scaling. This finding challenges the assumption that bigger always means more cells.
The fat body, which functions like both a liver and a fat storage depot in flies, provides another twist. Female fat cells are supersized, fueled by higher expression of ribosomal protein-coding mRNAs—molecular machinery that boosts protein production to support growth.
In contrast, males compensate with a greater number of smaller cells. This sex-specific balance between cell size and number not only explains organ scaling but also connects to broader metabolic differences, potentially influencing everything from energy use to lifespan.
Why it matters
What makes this work stand out is its novelty: It is the first to map allometric strategies at the cell-type level across multiple organs in Drosophila. By combining transcriptomic data with validation experiments, we showed that sex differences in anatomy and physiology are not quirks—they are developmental design choices.
Our results provide a framework to parse sexual size dimorphism into testable cellular components that can be mapped gene-by-gene and tissue-by-tissue. Because the Fly Cell Atlas offers a whole-animal reference, these rules can now be explored in other systems where sexual size dimorphism is common (including mammals), helping clarify whether sex-biased traits arise from proliferation programs, growth pathways, or both.
What surprised us
We expected one dominant mechanism. Instead, we found organ-specific strategies—even within a single metabolic tissue such as the fat body. The observation that the female fat body enlarges cells (with elevated ribosomal gene expression) while the male fat body increases cell counts suggests that distinct transcriptional circuits and growth control nodes are sex-tuned for each tissue, despite a shared genome.
This story is part of , where researchers can report findings from their published research articles. for information about Science X Dialog and how to participate.
More information: Soumitra Pal et al, Cell type specific allometry controls sex-differences in Drosophila body size, bioRxiv (2025). .
Data resource used: Hongjie Li et al, Fly Cell Atlas: A single-nucleus transcriptomic atlas of the adult fruit fly, Science (2022).
Soumitra Pal is a Staff Scientist at the National Eye Institute, National Institutes of Health (NIH), Bethesda, Maryland, U.S. He specializes in computational biology and bioinformatics, focusing on single-cell genomics and epigenomics. His research investigates how gene regulation and cellular diversity evolve across species, sexes, tissues, and disease states. By integrating large-scale single-cell datasets with cross-species genomic comparisons, he aims to identify conserved transcriptional programs and build computational frameworks to predict cell fate and function.
Soumitra is passionate about open science, reproducible research, and mentoring young scientists interested in computational genomics. Soumitra's long-term vision is to establish his own lab that combines AI-driven modeling with experimental validation to decode the rules of cellular identity and evolution.