New molecular strategy achieves complete synthesis of anti-MRSA natural product

Sanjukta Mondal
contributing writer

Stephanie Baum
scientific editor

Robert Egan
associate editor

Spiroaspertrione A is a complex polycyclic compound naturally produced by the fungus Aspergillus sp. TJ23. First isolated in 2017, it quickly drew scientific attention for its promising ability to combat drug-resistant bacteria and restore their sensitivity to existing antibiotics.
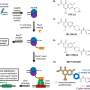
Scientists have now found a way to carry out the total synthesis of the molecule in 16 steps, starting from a chiral pool building block called (+)-enoxolone that costs less than one euro per gram. The synthesis technique is in Science.
Staphylococcus aureus (staph) is a type of bacteria that quietly lives on our skin and in our noses. It usually does no harm, but when it turns invasive, it triggers dangerous infections like sepsis, pneumonia, and many hospital-acquired infections. What makes it truly alarming is its growing resistance to antibiotics, which can turn treatable infections into deadly threats.
In 2021 alone, methicillin-resistant staph or MRSA led to 130,000 deaths worldwide. One promising way to fight antibiotic resistance is by using small molecules like (−)-spiroaspertrione A that can make MRSA sensitive to existing drugs again.
Ever since the discovery of its therapeutic properties, scientists have been attempting to develop effective strategies to synthesize the molecule in a laboratory.
The major bottleneck has been formation of the molecule's spirobicyclo[3.2.2]nonane core. To construct the spirobicyclic scaffold, quaternary centers must be formed at C8 and C2′. However, the highly functionalized nature of the polycyclic backbone that makes up the molecule forms a tight cage-like structure that prevents further modifications. It blocks chemical groups from accessing reactive sites and driving the reaction.
To overcome the structural hurdles, the researchers carried out a Diels–Alder cycloaddition followed by a key divinylcyclopropane rearrangement (DVCPR). Instead of adding groups to an already crowded backbone, they created a flexible precursor molecule and heated it to 180°C. This heating triggered the atoms to rearrange and form the cage-like spirobicyclo[3.2.2]nonane core in a single step, a mechanism further supported by density functional theory (DFT) calculations.
Once the core structure of the target molecule was in place, the researchers carefully added oxygen and other functional groups at precise positions. Through a series of oxidation reactions, they produced an aldehyde, which was then transformed into (−)-aspermerodione. When this compound was heated with a base, it underwent a molecular rearrangement that closed the ring and yielded the final product—(−)-spiroaspertrione A.
While the yield was 2.3%, which is quite low, the study was able to completely synthesize an anti-MRSA compound starting from an inexpensive, commercially available precursor.
The researchers noted that through the process, they were to gain deeper insight into the structures of other molecules within the natural product family. These findings could guide the design of new compounds capable of resensitizing antibiotic-resistant bacteria to existing drugs.
Written for you by our author , edited by , and fact-checked and reviewed by —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Wenbo Huang et al, The total synthesis of (−)-spiroaspertrione A: A divinylcyclopropane rearrangement approach, Science (2025).
Journal information: Science
© 2025 Science X Network