Novel phage DNA modifications offer new hope against antibiotic-resistant superbugs

Sadie Harley
scientific editor

Robert Egan
associate editor

Collaborating researchers have made a breakthrough discovery regarding the intricate defense systems of bacteriophages (phages)—viruses that can specifically target harmful bacteria without harming human cells and beneficial microbes.
The researchers from the Singapore-MIT Alliance for Research & Technology's (SMART) Antimicrobial Resistance (AMR) interdisciplinary research group, alongside the University of Otago and other collaborators from Nanyang Technological University (NTU Singapore), Delft University of Technology, University of Canterbury and Massachusetts Institute of Technology (MIT), found a novel type of phage DNA modification, with the addition of up to three arabinose sugars, which could help to protect phage DNA from being damaged and survive bacterial attacks.
This knowledge could be leveraged to develop new, targeted phage treatments for critical antibiotic-resistant pathogens.
Phages are a powerful weapon against the growing threat of antimicrobial resistance, as they have the potential to infect and kill bacterial strains that are resistant to antibiotics. For billions of years, bacteria and their viral predators—phages—have co-evolved in a complex evolutionary arms race.
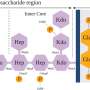
While phages outnumber bacteria by about a tenfold margin, bacteria have developed diverse defense systems to evade phage infection and killing. These defenses include well-known mechanisms like the restriction-modification (RM) and CRISPR-Cas systems, which can recognize and destroy invading phage DNA.
In response, phages have developed their own counter-defense strategies, including modifying their DNA to evade bacteria's diverse DNA-sensing and -targeting defenses.
In a paper titled "Phage arabinosyl-hydroxy-cytosine DNA modifications result in distinct evasion and sensitivity responses to phage defense systems," in Cell Host & Microbe, the researchers document the discovery of a new type of phage DNA modification with the addition of arabinose sugars to cytosine in the DNA via a unique chemical linkage, which can be further modified with one or two more arabinose sugars to form double or triple arabinosylated DNA through cellular processes.
The modifications with more sugars were found to provide greater protection against bacterial defenses. More importantly, many of these modified phages are targeting major pathogenic bacteria and show promise for developing new treatments against antibiotic-resistant bacteria, including Acinetobacter baumannii.
A. baumannii—classified as a critical priority in the World Health Organization (WHO) Bacterial Priority Pathogens List (BPPL) 2024—is a superbug that causes potentially life-threatening infections such as pneumonia, meningitis and sepsis, and urinary tract, blood and wound infections, especially among individuals with compromised immune systems. This bacterium is often resistant to multiple drugs, leaving few or no effective treatments.

"Our research has revealed that the interactions between phage and bacterium are much more complex than initially expected, and a better understanding of these interactions is key to using phages to fight bacterial infections," said Dr. Liang Cui, Principal Research Scientist at SMART AMR and co-corresponding author of the paper.
"Leveraging a highly sensitive analytical platform capable of detecting and identifying novel phage DNA modifications developed at SMART, we have been able to uncover a number of novel phage DNA modification systems and the findings in this publication are our most recent discoveries.
"We look forward to further studying how these tools and knowledge could help to better develop phage therapeutics against bacterial infections."
"Understanding the cellular processes where phages modify their DNA to defend themselves from bacteria, will allow the development of more effective phage therapeutics against antibiotic-resistant pathogens," said Prof Peter Fineran, Molecular Microbiologist and Head of the Phage-host interactions (Phi) laboratory at the University of Otago, and co-corresponding author of the paper.
"Through this work, we have also established methods capable of genetically engineering these phages with DNA modifications, which will help in their future development as therapeutics. It is exciting how much biological innovation the phage-bacterial 'arms race' has yielded, and our research is regularly gaining insight into this incredible diversity that can then be harnessed in biotechnological applications. It was fantastic to work with the excellent team at SMART."
This groundbreaking discovery enhances the field of phage therapy, enabling the development of more effective treatments against antibiotic-resistant pathogens.
By demonstrating that natural DNA modifications in phages occur at a much higher rate than previously predicted, the study not only improves the understanding of phage biology but also revises the fundamental understanding of phage biology, opening up new avenues for discovering other novel phage DNA modification systems.
This research was made possible by an interdisciplinary approach, combining the expertise of SMART AMR in analytics with that of its collaborators in informatics, genomics and molecular biology.
Moving forward, the team will focus on exploring the newly discovered diversity of these phage DNA modification systems, which will improve the understanding of the complex interactions between phages and bacteria, and ultimately help combat the growing threat of antimicrobial resistance.
More information: Marina Mahler et al, Phage arabinosyl-hydroxy-cytosine DNA modifications result in distinct evasion and sensitivity responses to phage defense systems, Cell Host & Microbe (2025).
Journal information: Cell Host & Microbe