Researchers reveal a surprising new role for a key protein in cell division

Sadie Harley
scientific editor

Robert Egan
associate editor

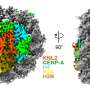
Scientists at the Ruđer Bošković Institute (RBI) in Zagreb, Croatia, have discovered that the protein CENP-E, long believed to act as a motor dragging chromosomes into place during cell division, in fact plays a completely different role in chromosome movement. It stabilizes the first attachments of chromosomes to the cell's internal "tracks," ensuring they line up correctly before being divided.
In a related study, scientists found that small structures inside our cells, called centromeres, which were once thought to function independently, help guide this key protein that ensures cells divide properly. The findings overturn two decades of textbook understanding and carry major implications for life sciences, since errors in this process underline many cancers and genetic diseases.
Every second, trillions of times over, your body pulls off something that's nothing short of miraculous. A single cell prepares to divide, carrying three billion letters of DNA, and somehow ensures both daughter cells receive perfect copies.
If that balance tips, the consequences are immediate and dire. A single misplaced chromosome can derail development, fuel infertility, or spark cancer. Cell division is one of biology's most unforgiving games.
For years, scientists thought they had identified at least one of its key players: CENP-E, described as a workhorse motor that hauls stray chromosomes into the center of the cell for orderly division. The story was neat, elegant, and wrong.
Two new studies from RBI, published in Nature Communications and led by Dr. Kruno Vukušić and Professor Iva Tolić dismantled that model and proposed new ways of its regulation.
Dr. Vukušić completed his postdoctoral training in an ERC Synergy team and is preparing to establish his own research group at RBI. Prof. Tolić, a cell biologist and head of the Laboratory for Cell Biophysics at RBI, is a member of EMBO and Academia Europaea.
Together, their expertise and vision drove this research, revealing that CENP-E is not the "muscle" of the operation, but the key missing regulator—the factor that flips the switch at the right moment, allowing the cellular choreography to unfold.
"CENP-E is not the engine pulling chromosomes to the center," Vukušić says. "It is the factor that ensures they can attach properly in the first place. Without that initial stabilization, the system stalls."

A city of infinite traffic
Imagine rush hour in the largest city. You can picture millions of cars, millions of intersections. One mistake can gridlock the entire system.
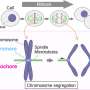
Now shrink that image to the micrometer scale of a cell. Chromosomes are trains, each one carrying DNA cargo. Microtubules, the thin fibers of the cell's skeleton, are the rails. For division to succeed, every train must lock onto the tracks coming from the right direction and line up at the central station.
The old model cast CENP-E as the locomotive, dragging stragglers into place. The Zagreb team found something subtler: CENP-E is not the train but the missing coupling element, the mechanism ensuring the hitch is strong enough to hold. Without it, trains stall at the edge of the station, unable to move forward.
When the lights refuse to change
Why do chromosomes hesitate at the edges? The answer lies in Aurora kinases, a family of proteins that act like overzealous traffic lights. They flood the cell with "red" signals, destabilizing early attachments and preventing chromosomes from locking on too soon in the wrong place.
This safeguard prevents errors near the poles of the cell but also risks producing too much red and not enough green. Here, CENP-E steps in. By modulating the signals, it eases the light to green just enough for chromosomes to catch hold. Once that first stable connection forms, the rest follows naturally: chromosomes align in the middle, guided by spindle geometry and microtubule dynamics.
"It's not about brute force," Tolić explains. "It's about creating the conditions for the system to run smoothly. CENP-E's key role is to stabilize the start, and once that happens, the rest of mitosis unfolds correctly."

A textbook story unravels
For nearly 20 years, biology textbooks taught the simpler story of CENP-E as a motor protein pulling cargo to the metaphase plate. The Zagreb study forces a rewrite.
"Congression, the alignment of chromosomes, is intrinsically linked to biorientation," says Tolić. "What we show is that CENP-E doesn't contribute significantly to the movement itself. Its crucial role is stabilizing end-on attachments at the start. That is what allows the system to proceed correctly."
It is a fundamental shift in framing: away from force and motion, toward regulation and timing. And that shift has consequences well beyond the classroom.
To outsiders, the distinction may seem subtle. In biology, details matter. Errors in chromosome segregation are a defining feature of cancer. Tumor cells are patchworks of duplications and deletions of entire chromosomes or their segments, each tracing back to a failure in the cellular traffic system.
By showing that CENP-E's primary role is to regulate the first attachments—and tying this regulation to Aurora kinase activity, the Zagreb team has not just linked two processes once thought to act independently but has mapped a critical vulnerability. This insight could inspire drugs that fine-tune the balance, suppressing runaway divisions or rescuing stalled ones.
"This isn't just about rewriting a model," Vukušić says. "It's about identifying a mechanism that directly links to disease. That opens doors for diagnostics and for thinking about new therapies."
The order in apparent chaos
At its heart, the discovery is about finding order in chaos. Each day, trillions of cells divide into the human body, each gambles against entropy. The work from Zagreb illuminates one of the hidden rules of that gambling. By redefining the role of CENP-E, and linking it to other processes inside cells, the team has given biology a clearer blueprint of how cells keep their traffic moving under impossible pressure.
"By uncovering how these microscopic regulators cooperate," Tolić says, "we are not only deepening our understanding of biology but also moving closer to correcting the failures that underlie disease."
More information: CENP-E initiates chromosome congression by opposing Aurora kinases to promote end-on attachments. Nature Communications (2025).
Kinetochore-centrosome feedback linking CENP-E and Aurora kinases controls chromosome congression. Nature Communications (2025).
Journal information: Nature Communications
Provided by Ruđer Bošković Institute