Bacteria reveal hidden powers of electricity transfer

Gaby Clark
scientific editor

Robert Egan
associate editor

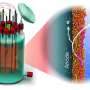
Microbes are masters of survival, evolving ingenious strategies to capture energy from their surroundings. For decades, scientists believed that only a handful of bacteria used specialized molecular "circuits" to shuttle electrons outside their cells—a process known as extracellular electron transfer (EET). This mechanism is critical for cycling carbon, sulfur, nitrogen, and metals in nature, and it underpins applications ranging from wastewater treatment to bioenergy and bioelectronics materials.
Now, KAUST researchers have discovered that this remarkable ability is far more versatile and widespread than previously imagined. The paper is in The ISME Journal.
Working with Desulfuromonas acetexigens—a bacterium capable of generating high electrical currents—the team combined bioelectrochemistry, genomics, transcriptomics, and proteomics to map its electron transfer machinery. To their surprise, D. acetexigens simultaneously activated three distinct electron transfer pathways previously thought to have evolved separately in unrelated microbes: the metal-reducing (Mtr), outer-membrane cytochrome (Omc), and porin-cytochrome (Pcc) systems.
"This is the first time we've seen a single organism express these phylogenetically distant pathways in parallel," says first author Dario Rangel Shaw. "It challenges the long-held view that these systems were exclusive to specific microbial groups."
The team also identified unusually large cytochromes, including one with a record-breaking 86 heme-binding motifs, which could enable exceptional electron transfer and storage capacity. Tests showed that the bacterium could channel electrons directly to electrodes and natural iron minerals, achieving current densities comparable to the model species Geobacter sulfurreducens.
By extending their analysis to publicly available genomes, the researchers identified more than 40 Desulfobacterota species carrying similar multipathway systems across diverse environments, from sediments and soils to wastewater and hydrothermal vents.
"This reveals an unrecognized versatility in microbial respiration," explains Krishna Katuri, co-author of the study. "Microbes with multiple electron transfer routes may gain a competitive advantage by tapping into a wider range of electron acceptors in nature."
The implications go well beyond ecology. Harnessing bacteria that can employ multiple electron transfer strategies could accelerate innovations in bioremediation, wastewater treatment, bioenergy production, and bioelectronics. For instance, electroactive biofilms like those formed by D. acetexigens could help recover energy from waste streams while simultaneously treating pollutants.
"Our findings expand the known diversity of electron transfer proteins and highlight untapped microbial resources," adds Pascal Saikaly, who led the study. "This opens the door to designing more efficient microbial systems for sustainable biotechnologies."
As researchers delve deeper into the microbial world, the discovery that a single bacterium can use multiple pathways underscores how much remains to be explored and how these hidden strategies could power a cleaner, more sustainable future.
More information: Dario R Shaw et al, Independently evolved extracellular electron transfer pathways in ecologically diverse Desulfobacterota, The ISME Journal (2025).
Journal information: ISME Journal