How chromosomes separate accurately: Molecular 'scissors' caught in action

Sadie Harley
scientific editor

Robert Egan
associate editor

Cell division is a process of remarkable precision: during each cycle, the genetic material must be evenly distributed between the two daughter cells. To achieve this, duplicated chromosomes, known as sister chromatids, are temporarily linked by cohesin—a ring-shaped protein complex that holds them together until separation.
Researchers at the University of Geneva (UNIGE), in collaboration with the National Cancer Institute (NCI) and the University of California, San Francisco (UCSF), have uncovered the mechanism by which separase—the molecular ''scissors'' responsible for this cleavage—recognizes and cuts cohesin.
published in Science Advances, shed new light on chromosome segregation errors that can lead to certain forms of cancer.
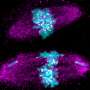
Before a cell divides, its chromosomes are duplicated. These identical copies, called sister chromatids, are held together by cohesin—a ring-like structure composed of several proteins that prevents premature separation.
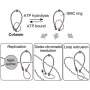
When the cell is ready to divide, separase, a specialized enzyme, cleaves one of the cohesin subunits, the protein SCC1, allowing the chromatids to separate and the genetic material to be evenly distributed between the two daughter cells. Any malfunction in this process can compromise genome stability, potentially resulting in severe diseases, including cancer.
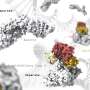
The team led by Professor Andreas Boland from the Department of Molecular and Cellular Biology, Faculty of Science, UNIGE, focuses on understanding how separase recognizes and cleaves its targets. In collaboration with the NCI and UCSF, the researchers resolved the structure of the complex formed between human separase and SCC1.
A first-ever structural map of human separase
Using cryo-electron microscopy (cryo-EM)—a cutting-edge technique that enables biological samples to be visualized in their native state at near-atomic resolution—the team captured the interaction between separase and SCC1 and identified the precise cleavage sites on the protein.
"While two separase cleavage sites had previously been identified in SCC1, our study identifies the correct location of these sites," explains Jun Yu, research and teaching fellow in the Department of Molecular and Cellular Biology at UNIGE and co–first author of the study.
Biochemical and structural analyses also revealed multiple "docking sites" on the surface of separase, ensuring high-affinity binding of SCC1 to separase prior to cleavage. These contact points include five phosphate-binding sites that recognize phosphorylated residues on SCC1.
"Our affinity experiments showed that these phosphate–separase interactions stabilize the complex and accelerate SCC1 cleavage, ensuring fast and precise separation of chromosomes," note Sophia Schmidt and Margherita Botto, a Ph.D. student and a postdoctoral researcher in Boland's group and co-authors of the study.
A step forward in understanding cell division disorders
"Our work provides an extensive functional and structural framework to understand how separase is regulated and how it recognizes its substrates," concludes Andreas Boland, who led the study.
These discoveries pave the way for future drug design studies aimed at controlling separase activity. The molecular insights provided by this study could ultimately lead to the development of specific inhibitors capable of blocking cohesin cleavage by separase and, consequently, uncontrolled cell division—a key feature of cancer development.
More information: Jun Yu et al, Substrate recognition by human separase, Science Advances (2025).
Journal information: Science Advances
Provided by University of Geneva