Iron core-shell catalyst boosts hydrogen economy of direct syngas to olefin conversion

Sanjukta Mondal
contributing writer

Sadie Harley
scientific editor

Robert Egan
associate editor

Scientists have catalyst that improves the typically low hydrogen atom economy (HAE) in the direct synthesis of olefins—small hydrocarbon molecules. It converts the water produced as a by-product into hydrogen for olefin production, thereby boosting overall efficiency.
Olefins derived from petroleum are the building blocks for many plastics and fuels. Direct conversion of syngas—a mixture of carbon monoxide (CO) and hydrogen (H2)—into olefins offers a promising alternative to reducing reliance on petroleum. It opens ways for using syngas derived from coal, biomass, or natural gas as a feedstock for olefin production.
In this study published in Science, researchers presented a sodium-modified FeCx@Fe3O4 core-shell catalyst produced via coprecipitation and thermal treatment. The catalyst achieved over 75% olefin selectivity and a 33% by weight hydrocarbon yield. It also had an HAE of ~66–86%, which is significantly higher than the ~43–47% seen in the traditional syngas-to-olefin (STO) conversion methods.

Hydrogen atom economy (HAE) measures how efficiently a reaction uses its hydrogen atoms to make the final product. A higher HAE means more product and less wastage. Traditional STO methods show low HAE for two main reasons. First, the direct conversion process produces water as a by-product, which removes hydrogen that could otherwise form valuable hydrocarbons, leading to low HAE.
Second, olefin synthesis typically requires syngas with an H2/CO ratio near 2:1, while syngas from coal and many other sources often have far less hydrogen (below 0.8:1). To compensate, industries use the water–gas shift (WGS) reaction to add more hydrogen. This step, however, comes at a cost: it lowers the overall HAE, makes olefin synthesis expensive and generates CO2 as a by-product.
Leveraging the by-products for better HAE
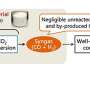
The researchers turned a problem into a solution. They coupled the water-gas shift reaction—which converts CO and H2O into CO2 and H2—directly with STO, creating a synergistic system that can increase the HAE of olefin production.
The newly developed catalyst, a sodium-modified FeCx@Fe3O4 core–shell nanoparticle, drove reactions in which the water produced by STO was immediately converted in situ to hydrogen, which in turn fueled further olefin formation.

Each layer of the core-shell nanoparticle had a specific role. The inner FeCx core catalyzed the STO reaction, converting syngas into olefins and producing H2O as a by-product. The water then diffused into the porous Fe3O4 outer shell, which kick-started the WGS reaction.
The H2O in the shell also reacted with the excess CO from the WGS reaction to produce additional H2 and CO2. The newly generated H2 was fed back into the STO pathway, reducing the need for external hydrogen and increasing the HAE in the process.
After testing the catalyst in fixed-bed reactors at a temperature and pressure of 623 K, 2 MPa, the researchers saw a >75% olefin selectivity with ~95% of CO was converted to the desired product. The hydrogen atom economy also rose to the ~66–86% range. The catalyst performance remained stable for 500 hours, and reduced waste generation per product by 46%.
The researchers note that the developed catalyst enables a WGS–STO coupling pathway that is more efficient and less environmentally costly. It delivers higher HAE while reducing steam usage, wastewater generation, and CO2 emissions, offering a sustainable alternative to current methanol-to-olefin processes.
Written for you by our author , edited by , and fact-checked and reviewed by —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Chang Gao et al, Conversion of syngas into olefins with high hydrogen atom economy, Science (2025).
Journal information: Science
© 2025 Science X Network