From coffee rings to saucer patterns—how graphene oxide's surface chemistry shapes evaporating droplet deposits

Lisa Lock
scientific editor

Andrew Zinin
lead editor

An evaporating colloidal particle–laden droplet leaves behind a ring-like residue after drying. We routinely observe this ubiquitous phenomenon for dried coffee drops; thus, it is known as the "coffee-ring effect." As a droplet evaporates, the edges dry faster than the center, pulling fluid—and suspended particles—outward. This creates a dense ring of material at the periphery of the droplet. It's a familiar sight to anyone who's spilled tea or coffee, but for scientists working on coatings and inks, this effect can be frustrating. In many applications, a uniform deposit is far more useful than a ring.
In our recent study in Langmuir, we explored what happens when tiny sheets of carbon—specifically, reduced graphene oxide (rGO) and graphene oxide (GO)—are suspended in water droplets and left to dry on a glass surface. The results were striking, and they offer new possibilities for printable electronics, coatings, and more.
Quasi-two-dimensional materials and their colloidal deposits
Quasi-two-dimensional carbon materials like graphene, reduced graphene oxide (rGO), and graphene oxide (GO) are at the heart of many emerging technologies due to their remarkable electrical, thermal, and mechanical properties. Despite their promise, there's a surprising gap in our understanding of how these materials behave when deposited from evaporating droplets—particularly how the level of oxidation affects the final dried patterns.
This matters because industries developing printable electronics, supercapacitors, and functional coatings rely on precise control over how these materials form films on surfaces. Our study set out to explore this issue by examining how rGO and GO—suspended in water droplets—leave behind different deposit patterns as they dry.
A key difference between rGO and GO lies in their surface chemistry. GO is highly oxidized and contains a variety of oxygen-rich functional groups such as hydroxyl, carboxyl, and carbonyl. rGO, on the other hand, has fewer oxygen groups and a more graphitic, less polar structure. These differences turn out to play a major role in how the materials move and settle during evaporation.
So we asked: Can we control the pattern left behind by tweaking the chemistry of the suspended quasi 2D materials?
GO vs. rGO: A surprising shift
When we used rGO—a form of graphene oxide that's been chemically or "green" reduced—we saw the usual coffee-ring pattern. But when we switched to GO, something unexpected happened. The ring disappeared, replaced by a smooth, even layer across the droplet's footprint. It looked more like a tiny saucer than a ring.
Why? The answer lies in GO's structure. At the heart of the underlying mechanism lies the self-assembly of GO at the liquid-vapor interface. The presence of strongly hydrophilic hydroxyl groups alongside the residual hydrophobic graphitic domains causes the GO-sheets to exhibit an amphiphilic nature. Consequently, they propel toward the liquid-vapor interface and anchor with it.
Furthermore, the GO sheets spontaneously align with each other, or self-assemble, as the adjacent GO-sheets are bound together via a hydrogen bonding network formed with the water molecules present in the dispersing aqueous medium. When we viewed the GO-laden droplet through a crossed-polarizer under a microscope, we observed interesting birefringent textures, which is a characteristic of GO lyotropic liquid crystals (GOLLCs), confirming the self-assembly.
The so-collected GO-sheets at the interface thus descend with the interface as the evaporation proceeds and at last they deposit uniformly onto the underlying surface that looks like an inverted saucer.
Green chemistry bonus
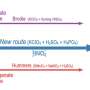
As a bonus, we demonstrated an eco-friendly route to produce rGO using Acacia concinna seed extract. This "green" synthesis performed as well as the traditional method using hydrazine—without the toxic chemicals. We found that both forms of rGO created the same coffee-ring deposits, underscoring the broader point: It's the oxygen content—not the synthesis route—that governs the deposit pattern. The green synthesized rGO possesses the same properties as those of hydrazine-reduced rGO, thus is a greener, yet prominent candidate to meet industrial demands.
Controlling droplet drying patterns is important for technologies like inkjet printing of electronics, biosensors, and coatings. Our findings show that by tuning surface chemistry—specifically the oxygen content of graphene-based materials—we can control where particles end up after evaporation.
This opens the door to greener, more predictable, and tunable printing processes, using materials as simple as plant extracts.
This story is part of , where researchers can report findings from their published research articles. for information about Science X Dialog and how to participate.
More information: Yogita et al, Self-Assembly and Deposits of Reduced Graphene Oxide and Graphene Oxide Colloids from Desiccating Aqueous Sessile Droplets, Langmuir (2025).
Journal information: Langmuir
Yogita is a postdoctoral fellow in the ������Ƶics Department, DIT University, Dehradun, India. Sanjeevni Rana is an undergraduate student and Kajal Sharma is a PhD scholar in the same department. Ravi Kumar Shukla is a professor and Sanghamitro Chatterjee is an assistant professor in the same department. Bibek Kumar is a PhD scholar in the Mechanical Engineering department of IIT Bombay, Mumbai, India. Rajneesh Bhardwaj is a professor in the same department.